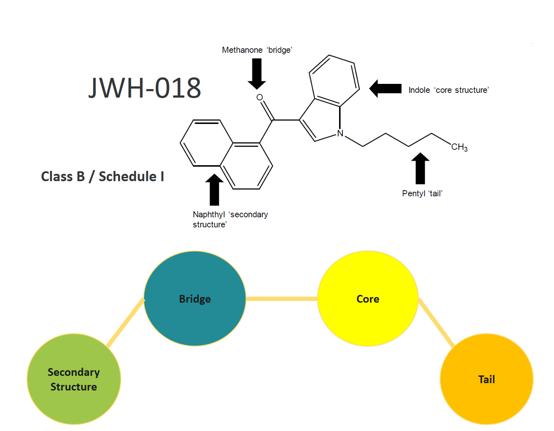
In 2016 the UK government amended the 1971 Misuse of Drugs Act (MoDA) and added a section designed to control the newer synthetic cannabinoids in increasing supply on the black market. Unfortunately, the definition was drawn extremely broadly
Pharmaceutical companies and other life science R&D organizations routinely work with controlled substances, narcotics and psychotropic drugs, and must have adequate controls in place to meet the legislative requirements of the countries in which they operate. Today these organisations hold and dispense tens of millions of samples in highly automated processes making manual compliance activities impractical.
The impact of this amendment is that typical Pharmaceutical libraries have seen their controlled compounds rise into the tens of thousands, with the clear majority having no indication of CB1 activity. This has prevented hit ID and lead optimization activities and limited collaborative research due to the inability to ship these compounds outside the UK. Many existing products like Atorvastatin (Lipitor – Pfizer, statin) and Lostartan (Cozaar – Merck & Co, Angiotensin) are also affected. Not only are these proven medicines, and so do not fall into Schedule 1, which is specifically for substances considered by the government to have no medicinal value, but they also do not exhibit any cannabinoid-like activity
The Controlled Substance Compliance Expert Community is working with the UK’s Advisory Council for the Misuse of Drugs on mitigation options. We have provided expertise to narrow the scope of the definition and suggest other measures such as de-minimis limits and research exemptions.
Zofia Jordan will be speaking about the outcome at the Pistoia Alliance’s London conference in March.
